only return atoms within sphere=1 layer during generating HOSE code
🐛 Bug
by getSpheres(paras ) to get all atoms according with specified sphere,but only return sphere=1 atoms without more ones.
To Reproduce
Steps to reproduce the behavior:
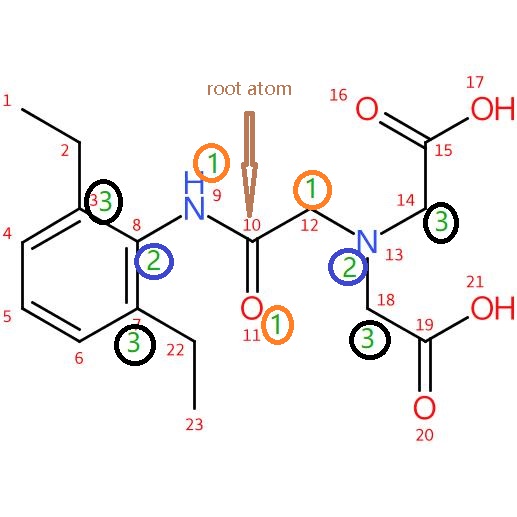
` String smiles = "CCC1=CC=CC(=C1NC(=O)CN(CC(=O)O)CC(=O)O)CC";//-----Universal SMILES
int sphere = 6;//----sphere
HOSECodeGenerator hcg = new HOSECodeGenerator();
IChemObjectBuilder bldr = SilentChemObjectBuilder.getInstance();
SmilesParser smipar = new SmilesParser(bldr);
IAtomContainer mol = smipar.parseSmiles(smiles);
List<IAtom>[] nodeAtoms = hcg.getSpheres(mol, mol.getAtom(9), sphere, false);
for (int i = 0; i < sphere; i++) {
for (IAtom iAtom : nodeAtoms[i]) {
System.out.println(iAtom.getSymbol());
}
System.out.println("-------");
}`
Environment
- OS: win10
- IDE: IDEA 2021
- JDK:1.8
- CDK version:2.8
Thanks please note hose codes are an outdated method and something like Signatures/CircularFingerprint is much better.
John, thanks for your valuable advice. It is mostly like that CircularFingerprinter could not be "reversed" to corresponding molecule structure as I wanted. Signatures could satisfy my requests. But how to convert atom index into vertexIndex in the job of calling this method signatureStringForVertex(vertexIndex, height) , I could not figure out a way for this conversion from CDK JavaDoc. Signatures usually gets rid of aromaticity/chirality infomation when "cutting" a fragment from a full molecule. Is there a straightforward strategy to recover all the lost info from signatures? All info play key role in AI modelling.
You should be able to get the atom info from the circular fingerprint… but I need to check.
Anyways the main point was you probably don’t want hose codes :)
key properties such as aromaticity, charge,stereotypical type etc. as well as height/sphere stored in a substructure based on CircularFP are highly preferred.